Abstract
Background: There are limited data on real-world treatment patterns and outcomes for follicular lymphoma (FL) in the relapsed/refractory (r/r) setting, with shorter response durations reported after each relapse (Link et al, 2019; Rivas-Delgado et al, 2019 and Batlevi et al, 2020). We examined treatment patterns for patients with FL initiating third line (3L) therapy at a single institution by time period in the post-rituximab era (2004-2010 and 2011-2020), and clinical outcomes for the overall cohort receiving therapy between 2004 and 2020.
Methods: This is a retrospective, observational study of patients with FL who initiated 3L therapy between 2004 and 2020 in routine clinical practice at The Christie NHS Foundation Trust, UK. We selected patients aged ≥18 years at 3L initiation, with histologically documented FL Grade 1−3a treated with two prior lines of systemic therapy including an anti-CD20 monoclonal antibody and an alkylating agent, and at least one year of follow-up after initiating 3L therapy; follow-up ended June 2021. We excluded patients with grade 3b FL or transformation to high grade lymphoma any time before 3L treatment. Overall response rate (ORR) and complete response (CR) to 3L therapy was calculated, and overall survival (OS), progression free survival (PFS) and time to next treatment (TTNT) were estimated using the Kaplan-Meier (KM) method with 3L therapy initiation date as the index date.
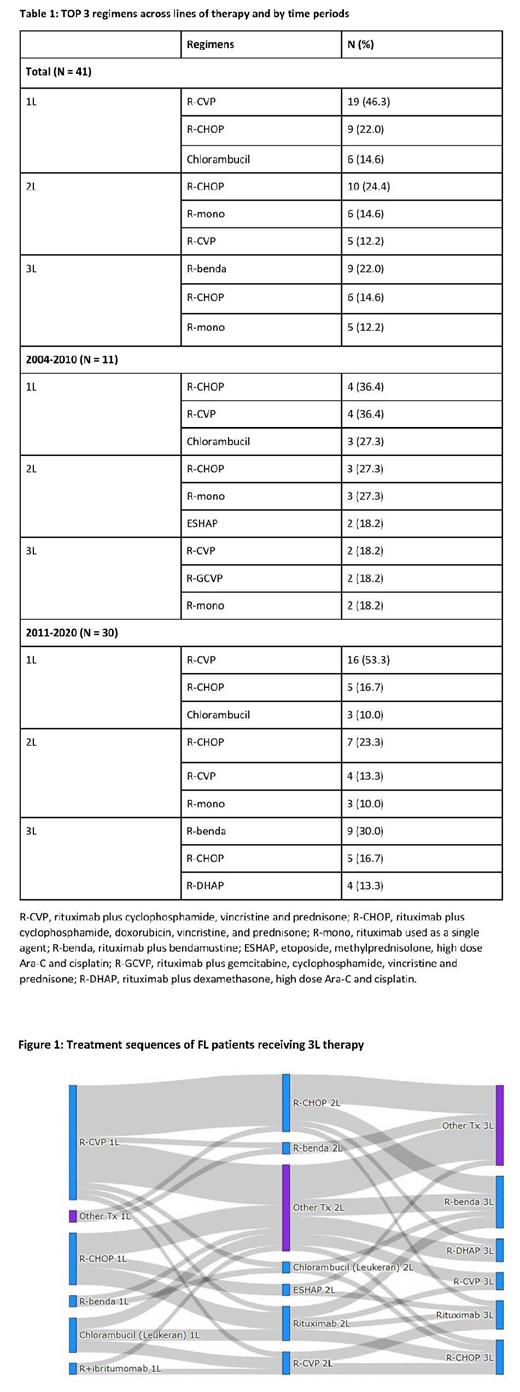
Results: Overall, 41 patients met all eligibility criteria; 11 and 30 patients received 3L therapy between 2004-2010 and 2011-2020, respectively. Median age at index date was 59 years and 53.7% were male; 73.2% had grade 1 or 2 FL; 78.1% had advanced stage (III/IV) FL at diagnosis. Median follow-up was 33.9 (IQR: 14.5, 63.0) months, and median time from diagnosis to 3L treatment was 60.2 (IQR: 29.4, 89.1) months. The most common regimen in 3L was rituximab plus bendamustine (R-benda) followed by rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and rituximab used as a single agent (R-mono). Treatment patterns differed by time period (Table 1). R-benda was more commonly used between 2011 and 2020. The most common sequence was rituximab plus cyclophosphamide, vincristine and prednisone (R-CVP) followed by R-CHOP and R-benda (Figure 1). ORR to 3L treatment was 61.0%, CR 29.3%. Median OS, PFS and TTNT with 95% confidence interval (CI) were 70.0 (30.2-NR), 19.2 (9.5-34.7) and 11.8 (9.0-27.6) months after 3L initiation, respectively. Two- and five-year OS rates were 79% and 50%, and two-year PFS rate was 37%.
Conclusions: Patients with r/r FL treated in the routine 3L setting have highly variable treatment patterns and unfavorable outcomes, representing a continued unmet medical need. This study is limited by its small size and evolving treatments, warranting a larger study of more recently treated 3L patients to evaluate the impact of modern treatment pathways and novel therapies on clinical outcomes for r/r FL.
Linton: University of Manchester: Current Employment; BeiGene: Research Funding; Hartley Taylor: Honoraria; Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Aptitude Health: Honoraria; Celgene: Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Julian: Genentech, Inc.: Current Employment, Current holder of stock options in a privately-held company. Gibb: The Christie NHS Foundation Trust: Current Employment; Takeda: Honoraria, Research Funding, Speakers Bureau. Li: Genesis Research: Current Employment. Liu: Genesis Research: Current Employment. Shewade: Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Radford: BMS: Honoraria; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; ADC Therapeutics: Consultancy, Current holder of individual stocks in a privately-held company, Honoraria, Speakers Bureau; AstraZeneca: Current holder of individual stocks in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal